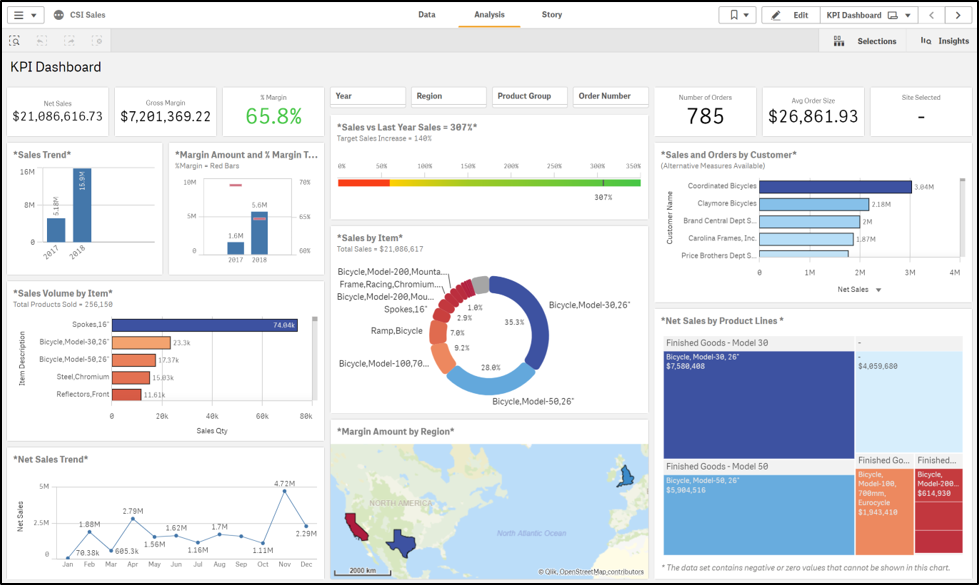
If you’ve been following the steps outlined in our previous blogs, then your journey to ERP selection is nearly complete. You’ve gathered an ERP selection team to discuss your business drivers and software needs. You’ve analyzed the cost of ERP ownership versus the gains. With these thoughtful conversations behind you and comprehensive reports in hand, you are prepared to make the biggest decision of all: choosing an ERP solution.
Read More
Insights to guide your digital transformation.
The ERP Selection Process: Some Assembly Required: Finding the Perfect ERP Partner
Aug 16, 2021 9:30:00 AM
The ERP Selection Process: Some Assembly Required: Understanding your ERP’s Value
Jul 26, 2021 8:00:00 AM
After your ERP selection team has factored the cost of implementing a modern ERP system, it is time to assess its value. As discussed in the previous blog, without this key piece of information for the decision-makers, all your ERP selection team’s hard work may be for naught.
Read MoreThe ERP Selection Process: Some Assembly Required: Determining the Costs of Your ERP System
Jul 12, 2021 8:05:00 AM
You have your power team, you’ve determined your top business drivers, and you’ve even developed your short list of business needs your ERP system must meet. What next? Time to factor in the cost versus the value of a new ERP system.
Read MoreThe ERP Selection Process: Generating The Short-List
Jun 22, 2021 7:30:00 AM
Now that your ERP selection power team has identified your top-tier business drivers, what’s next? The good news is that you’re halfway to the finish line, and you’ve successfully set yourself up for the next step: generating and prioritizing the short list to help you select the best ERP system to meet your established goals.
Read MoreThe ERP Selection Process: Building Goals Based on Your Business Drivers
Jun 8, 2021 8:00:00 AM
Now that you’ve built your power team for the ERP selection process, what’s next? Time to begin building your case. And to do that, you must first establish ERP goals based on your specific business needs. It is important to envision how the technology can be leveraged to improve activities not only within your business but also across the ecosystem your business serves.
Read MoreThe ERP Selection Process: Some Assembly Required
May 24, 2021 7:00:00 AM
Building a Power Team: The first major step in the journey toward adopting a new ERP system for your organization is assembling an effective project team. A well-appointed team can help you not only select the best possible ERP system suited to meet your organization’s needs but also mitigate employee resistance and secure leadership buy-in.
Read MoreTrending: Prescriptive Analytics Solving Business Problems with Minimal to No Side Effects
Apr 21, 2021 8:11:41 AM
Real-time data and predictive analytics are the talk of the town, but what if we could see into the future? Prescriptive analytics moves us several steps beyond predictive, allowing businesses to analyze possible outcomes of future decisions before they are even made.
Read MoreI’ll Take My Data To-Go, Please: Mobile BI in 2021
Apr 6, 2021 7:36:03 AM
Mobile Business Intelligence: The New Normal
The Mobile business intelligence (BI) market value is expected to top $20 billion by 20241. And no wonder; we are a mobile working population, glued to our phones and now, even to our watches. But we’ve been programmed to expect that everything on our phones provides us instant gratification, yet mobile BI apps have long operated very differently to that.
Read MoreWhat’s Trending in 2021: Will AI Render the Dashboard Obsolete?
Mar 18, 2021 4:33:28 PM
Imagine a future where the dashboard is not the first-place executives go after they pour themselves a cup of coffee. Although dashboards will never truly disappear, artificial intelligence (AI) is on a mission to fundamentally transform them, and perhaps one day, render them obsolete.
Read MoreThree BI Trends for 2021: An Overview
Mar 3, 2021 8:46:58 AM
If anything good came out of 2020, it’s this: adversity can speed up innovation years, or even decades, ahead of schedule. Technologies that were deemed futuristic and advanced, such as highly interactive videoconferencing and uninterrupted wireless networks, are now as mainstream as electricity. Each day, new technological breakthroughs occur, and it seems no sooner than a business embraces one, better ones show up the next day.
Read MoreMoving Your Business Intelligence to the Cloud
Jan 25, 2021 12:44:02 PM
According to Forbes magazine, “in the next 18 months or so, we’ll continue to see a huge acceleration in deploying or migrating databases to the cloud, reaching 75% by 2022.” Business intelligence solutions in the cloud represent the merging of two key trends—the progression of cloud computing as a cost-effective, agile platform for business applications and the use of BI technology to gain insight and improve the quality and speed of decision making.
Read MoreFDA/Regulated? Don’t Be Scared of the Cloud
Jan 12, 2021 9:23:14 AM
Most FDA/Regulated industries have taken a conservative approach to adopting innovative technologies, and given the high-demand environment in which they operate, their concerns are valid. But cloud-based software has come a long way in meeting the rigorous demands of regulated industries, and as a result, moving to the cloud can prove to be one of the best resolutions you make for the year 2021.
Read MoreJan 4, 2021 10:08:52 AM
Ready to upgrade or implement a new enterprise resource planning (ERP) solution? One of your initial decisions will be whether you want to deploy the solution on premise or in the cloud. Because The Copley Consulting Group offers Infor’s CloudSuite Industrial (CSI)/Syteline in either the cloud or in an on premise, we can provide key insights into the advantages and disadvantages of each option. So, which is best for your organization? First, let’s learn the key differences in each deployment option and how each should factor into your final decision.
Read MoreDec 4, 2020 11:18:28 AM
Think of all the reasons your business hasn’t moved to the cloud. Too difficult? Too costly? Too risky? Every industry has pockets of cautious executives who have yet to hitch their wagons to innovative concepts like cloud-based solutions, believing the risks still outweigh the benefits. Sticking with an on-premise solution may feel like an option free of risk, but that decision may slow down your company’s progress. It’s time to further investigate your reasons for holding back.
Read MoreBig Data and Small Data: A Collaborative Affair. “The Human Element: Building Bridges”
Nov 3, 2020 11:46:56 AM
You may have heard the saying, “big data” is about machines, while “small data” is about people, and the saying still has merit. Yes, big data can be quite distracting: it’s diverse, it’s constantly flowing, and it contains incredibly helpful information to guide and support your business objectives. But don’t let it pull you entirely away from ultimately what’s important, the human element.
Read MoreBig Data, Small Data or Both? A Collaborative Affair: Volume Versus Value
Oct 7, 2020 8:49:38 AM
There’s Gold in Them Hills! Mining Big Data for Value, Literacy and Sense
Think of big data as a rocky mountain, looming over your business, housing tons of valuable ore. You know the ore is there, but not how to find it. Mining big data for valuable and actionable small data poses a challenge for many SMBs and manufacturing companies, and there are many “mining packages” out there promising they are the best at data exploration and extraction. So, when it comes to approaching your mountain, how do you proceed? The best approach is the same approach a mining company utilizes to extract ore from a mountain: in well-planned phases.
Big Data and Small Data: A Collaborative Affair. Keeping up with the Bezoses
Sep 22, 2020 1:28:45 PM
Ask Amazon CEO Jeff Bezos what he has for breakfast, and he would probably reply, “big data.” Yes, big data is the Wheaties of Champions, and businesses are investing in the best tools and expertise to harness it. But not every business behaves like Amazon, and there’s one important tool that’s being bypassed on the “cereal shelf,” small data.
Read MoreJun 22, 2020 8:00:00 AM
Two questions your Board will ask the C-Suite about IT systems that your CIO must be prepared to answer:
1: Is our system secure?
2: If our system is breached, can we quickly get back up and running?
Eric Yuan, CEO and founder of Zoom Video Communications, knows firsthand the answers to these two questions after his video meeting platform was plagued with cyberattacks. Soon, “Zoombombing” became a common catchphrase, and the company raced to seal up security leaks, improve encryption, and destroy bugs in the software.
Read More5 Key Strategies to Develop a Dynamic Relationship with Your Data
Jun 15, 2020 8:00:00 AM
Every relationship needs to be nurtured, including the one you have with your data, and a full modernization of your legacy software is the first step. But regardless of where you are in your modernization journey, one key system that must be upgraded is your analytics platform. This platform behaves like a third-party, non-biased interpreter of your data, able to mine relevant and sometimes hidden information from all areas of your business, ultimately providing you with actionable solutions. Relationships with humans can be messy and hard to interpret, but a healthy relationship with your data shouldn’t be, especially with these 5 key strategies:
Read MoreWhat Do You and SpaceX Have in Common?
Jun 8, 2020 8:00:00 AM
In a world that is filled with disastrous news, for 10 seconds, all eyes were locked onto the sky as we watched the SpaceX Falcon 9 rocket booster launch two astronauts to the stars, and for those brief moments, we realized that humanity was always built for something bigger.
In truth, the SpaceX launch was “a welcome reminder of America’s global pre-eminence in science, technological innovation and private enterprise at a time its prospects and ambitions have been clouded by the coronavirus pandemic, economic uncertainty and political strife.” (NY Times)
Finding Our Pace Again: Evaluating & Adapting to Our Transformed Work Landscape
Jun 1, 2020 9:45:00 AM
Even before the pandemic, the fast-paced, rapidly-changing dynamics of our business world was enough to make us feel like Olympic runners, sprinting at high speeds simply to maintain our competitive edge. But add a mandated quarantine, a complete shift to WFH, and many of us have, as runners would describe it, “hit a wall.” Some of us have also lost our bearing, not even recognizing the track anymore. If we could stop to assess our situation, we would reach one realization we must accept: the landscape of our work lives has altered completely, perhaps forever, and discovering innovative software to chart a new course for our business has become our top priority.
Read MoreFinding New Opportunities with The Internet of Things (IoT)
Feb 25, 2020 8:26:08 AM
The Internet of Things (IoT) may sound like a far-off concept, but many manufacturers today, including small and midsize businesses (SMBs), are beginning to integrate automation and IoT technologies to work smarter for their customers. From usage stats to error logs to inventory, equipment outfitted with data-capture technologies collect and transmit insights that can be immensely valuable to manufacturers looking to gain an edge in 2020. Here’s how.
Read MoreCloud-Based ERP – A Gateway Technology for Digital Transformation
Feb 19, 2020 10:55:15 AM
It’s unusual to read anything about the manufacturing industry these days that doesn’t mention “digital transformation” and the “cloud.” Manufacturers are adopting sophisticated new tools to revolutionize their supply chains and optimize their processes. While some small and midsize manufacturers may not have a desire or need to dedicate significant capital to a completely digitized supply chain, many are taking steps to modernize their operations through sensible investments in new technologies.
Read MoreEmpowering Employees to Work Smarter with Analytics
Feb 10, 2020 10:30:00 AM
As discussed in the first blog in this series, manufacturing has seen a slowdown over the last year, both in terms of demand and job growth. It’s hard to know exactly when a recession will occur, but when faced with signs of a slowdown, manufacturers are wise to be proactive in evaluating their operations before action must be taken.
Read MoreAn Outlook for SMB Manufacturers
Jan 28, 2020 8:08:32 AM
Ask a room of manufacturers about their organizations’ goals for 2020, and you’ll find that target numbers may be more modest than in years past.
This cautious outlook is largely driven by the global purchasing manager’s index (PMI), which, in 2019, saw several consecutive months below 50.0, the level that distinguishes between expansion and contraction. At year’s end, U.S. manufacturing activity fell to 47.2 – the lowest level seen since June 2009, when the U.S. economy was nearing the end of the Great Recession.
Read MoreImplementing ERP in an FDA-Regulated Industry – What to Expect
Jan 14, 2020 10:21:33 AM
Growing Life Sciences manufacturers need systems and solutions in place that help them remain compliant, while helping them grow their business. Implementing an ERP system to meet business requirements that is validated in accordance with FDA regulations is a continual challenge. Finding an ERP solution provider that best suits your needs and has experience developing and deploying an FDA-Regulated ERP solution can be another challenge.
Read MoreGoing Global in a Regulated Industry: How Your ERP System Can Help
Dec 4, 2019 9:00:00 AM
As an early-stage life sciences manufacturer, you may have plans to release your product to the global market. In order for it to be commercially available on an international scale, approvals from more than one regulatory authority will be required.
Read MoreHow Embedded Data Elevates Analytic Capabilities for Manufacturers
Nov 18, 2019 3:00:00 PM
The impact of intelligence increases substantially when technology delivers valuable data - fast. Managers get instant access to information that they can use to make decisions on the fly to improve manufacturing processes.
Read MoreUsing ERP to Prime Your Life Sciences SMB for Investment
Nov 11, 2019 9:00:00 AM
When it comes to courting investment, there is no foolproof recipe for the success of a small to midsize Life Sciences firm. Your path may look very different from your peers’, and why shouldn’t it? As a unique organization with a distinct offering, setting yourself apart is critical to attracting the right investor.
Read MorePredictive Analytics Empowers Manufacturers to Make Informed Decisions
Nov 5, 2019 10:37:22 AM
In recent blogs we explored how analytics in manufacturing is more effective when delivering insights focused on specific industries, and when it’s easy to run a custom analysis to derive an answer to a certain question. Let’s now consider predictive analytics, which go a long way in helping manufacturers make accurate predictions, and then make decisions based on that window into the future.
Read More3 Reasons Growing Life Sciences Companies Should Adopt ERP Software
Oct 29, 2019 9:00:00 AM
Whatever your goal as a growing life sciences business, be it long-term profitability, FDA approval or acquisition, a lynchpin of your success will be learning to juggle short-term scalability while keeping your eye on the ball. The many tools and features of an enterprise resource planning (ERP) system can help small and midsize businesses (SMBs) in FDA-regulated industries organize and automate their operations.
Read MoreGrowing & Improving SMB Manufacturing Through Proper Planning
Oct 22, 2019 9:00:00 AM
Market growth drives the success of a small and medium sized business (SMB) manufacturers. It’s not necessarily by what their business leaders plan. Product demand is rising, and in order to handle that rising demand, businesses need more capacity and are adding shifts and additional facilities to their small business.
Read MoreWhy Associative Database Engines Are the Key to Uncovering Business Intelligence
Oct 8, 2019 9:04:32 AM
It’s a common request. Make data analysis easy to leverage so people can continually improve operations and forecast supply and demand. That’s what IT teams at manufacturing organizations keep hearing from plant managers, sales teams, and executives. That’s not all. They also want the data in real-time to be able to make informed decisions on the fly.
Read MoreKey Characteristics of SMB Manufacturers – Part 2
Sep 30, 2019 8:22:00 PM
As we mentioned in Part One of our Top Key Characteristics of SMB Manufacturers blog, small and midsize manufacturers have some key strengths that make them ideally suited for the digital era. Often categorized as insignificant, small to mid-sized businesses (SMBs) are often and mistakenly overlooked.
Read More